Chilo partellus (Swinhoe)
Common name: Spotted stalk borer; pink borer.
Taxonomic placing: Insecta, Holometabola, Lepidoptera, Crambidae.
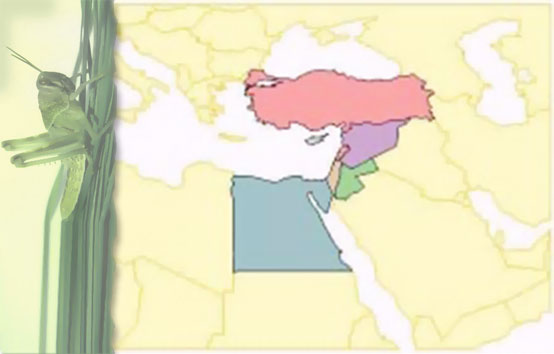
Geographical distribution: East and South Africa, The Middle East, India and Pakistan.
host plants: Several Poaceae (Gramineae), especially corn (maize), sorghum, and rice.
Morphology: The larvae (caterpillars) are yellow to pink with dark spots and four brown longitudinal stripes along the back, and a thoractic shiny brown plate. Body about 25 mm long, with prolegs that bear hooks arranged in a circle. The female forewings are yellow with dark longitudinal stripes, the hindwings almost white. Males are smaller and darker than females and their forewings are pale brown.
Life history: Young larvae feed on the leaves, then tunnel into the stems, wherein they feed and reach the growing point, which they damage, causing the characteristic “deadheart” symptom. They develop in about 4 weeks at 26–30°C and 60–80% RH, and pupate in the stems. The adults live only for 1-2 weeks. Under favorable conditions the pest can reproduce (laying 400-500 eggs/female) and develop all year-round, but during dry seasons larvae may enter diapause for several months and pupate with the onset of rain. Adults rest on plants during the day, being active at night.
Economic importance: Damage to maize and sorghum often exceeds 50-70% of the yield. Infestation can start around 2 weeks after seedling emergence. The larvae gnaw characteristic windows (“pinholes”) in host leaves, or mine therein, causing yellow streaks. Later they move down and rapidly bore into maize cobs and stems, tunnel therein and feed on the growing points, causing their death (“deadhearts”). This borer is a highly invasive pest and can fully or partially displace other borer species.
Management
Monitoring: During the vegetative growth stage, small larvae occur mostly behind leaf sheaths and in whorls in maize and in sorghum. In reproductive and senescent maize, small larvae also occur in ears, whereas elder larvae also in ears. In sorghum later stages are mostly found in stems. Infestations can be detected by walking through crops looking for the characteristic irregular shaped pinholes or shot holes, caused by young larvae, which may later change to elongated lesions and look for the presence of deadhearts. Samples of infested stems can be cut open to find caterpillars and pupae.
Horticultural control: Intercropping or mixing maize with non-host crops can reduce pest populations. Trap plants, such as Napier grass (Pennisetum purpureum Schumach) can be used, especially when grown around fileds. Such plants draw the female away from the crop and cause it to lay eggs on the trap, leading to poor larval development (the “push-pull” method). Destruction of all infested maize residues which might contain diapausing larvae. Crop rotation and refraining from growing susceptible crops in formerly-infested fields for several months. Grass weeds that could serve as alternate hosts should be also be destroyed.
Genetic manipulation: Sorghum plants transformed to express the toxic synthetic Cry1C gene were highly resistant to C. partellus, causing 100% mortality. Similar results were obtained with Bt-maize containing the Cry1Ab and Cry1Ba toxins.
Plant resistance: Fewer borer larvae that fed on indigenous (“landrace”) maize varieties survived as compared to hybrid maize, showing some resistance to the pest. Borer-resistant varieties of sorghum have been developed.
Biological control: Several indigenous and introduced parasitoids were assayed against the pest in South Africa but had little effect, probably due to unfavorable climatic conditions and the pest’s diapause. The gregarious endoparasitoid Cotesia flavipes Cameron (Braconidae) has been introduced into eastern Africa and became established there. Another endoparasitoid, Dentichasmias busseolae Heinrich (Ichneumonidae), attacked about 23% of the pest on maize and an average of 36% on sorghum. Applications of the entomopathogenic fungi Beauveria bassiana (Bals.-Criv.) Vuill. and Metarhizium anisopliae (Metchnikoff) Sorokin at concentrations, 1 × 108 conidia/ml, killed all second and third instar larvae of C. partellus within 2 weeks, the insects also showing a great reduction in food consumption.
References
Bahana, J.W. 1990. Bioecological studies on Dentichasmias busseolae Heinrich and its potential for biological control of Chilo partellus Swinhoe. International Journal of Tropical Insect Science 11: 765-772.
Ben-Yakir, D., Chen, M., Sinev, S. and Seplyarsky, V. 2013. Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) a new invasive species in Israel. Journal of Applied Entomology 137: 398–400.
Berger, A. 1989. Egg weight, batch size and fecundity of the spotted stalk borer, Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae), in relation to weight of females and time of oviposition. Entomologia Experimentalis et Applicata 50: 199-207.
Ignacimuthu, S. and Premkumar. A. 2014. Development of transgenic Sorghum bicolor (L.) Moench resistant to the Chilo partellus (Swinhoe) through Agrobacterium-mediated transformation. Molecular Biology and Genetic Engineering 2:1. http://dx.doi.org/10.7243/2053-5767-2-1.
Kfir, R., Overholt, W.W., Khan, Z.R. and Polaszek, A. 2002. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annual Review of Entomology 47: 701–731.
Midega, C.A., Khan, Z.R., Pickett, J.A. and Nylin, S. 2011. Host plant selection behaviour of Chilo partellus and its implication for effectiveness of a trap crop. Entomologia Experimentalis et Applicata 138: 40-47.
Mutyambai, D.M., Midega, C.A., Bruce, T.J., van den Berg, J., Pickett, J.A. and Khan, Z.R. 2014. Behaviour and biology of Chilo partellus on maize landraces. Entomologia Experimentalis et Applicata 153: 170-181.
Overholt, W.A., Ogedah, K. and Lammers. P.M. 1994. Distribution and sampling of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) in maize and sorghum at the Kenya coast. Bulletin of Entomological Research 84: 367-378.
Overholt, W.A. (and 6 co-authors): 1997. A review of the introduction and establishment of Cotesia flavipes Cameron in East Africa for biological control of cereal stemborers. Insect Science and Application 17: 79-88.
Sharma, H.C., Dhillon, M.K., Pampapathy, G. and Reddy, B.V.S. 2007. Inheritance of resistance to spotted stem borer,Chilo partellus, in sorghum, Sorghum bicolor. Euphytica 156: 117-128.
Tamiru, A., Getu, E., Jembere, B., and Bruce, T. 2012. Effect of temperature and relative humidity on the development and fecundity of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae). Bulletin of Entomological Research 102: 9-15.
Tende, R.M., Mugo, S.N., Nderitu, J.H., Olubayo, F.M., Songa, J.M and Bergvinson, D.J. 2010. Evaluation of Chilo partellus and Busseola fusca susceptibility to delta-endotoxins in Bt maize. Crop Protection 29:115-120.
Tefera, T. and Pringle, K.L. 2004. Mortality and maize leaf consumption of Chilo partellus (Lepidoptera: Pyralidae) larvae infected by Beauveria bassiana and Metarhizium anisopliae. International Journal of Pest Management 50: 29-34.
Unnithan, G.C. and Saxena, K.N. 1990. Population monitoring of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) using pheromone traps. International Journal of Tropical Insect Science 11: 795-805.